Market Overview:
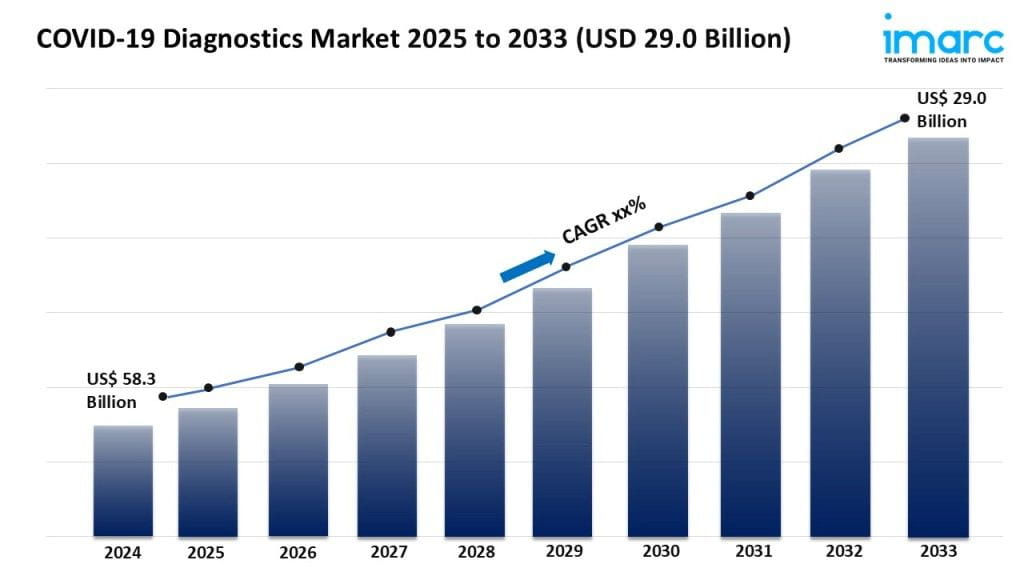
- The global COVID-19 diagnostics market size reached USD 58.3 Billion in 2024.
- The market is expected to reach USD 29.0 Billion by 2033, exhibiting a growth rate (CAGR) during 2025-2033.
- North America leads the market, accounting for the largest COVID-19 diagnostics market share.
- Instruments account for the majority of the market share in the product type segment due to their simplicity, functionality, and aesthetic appeal.
- Nasopharyngeal swabs hold the largest share in the COVID-19 diagnostics industry.
- Molecular (RT-PCR) testing remains a dominant segment in the market.
- Non-point-of-care (non-PoC) represents the leading mode segment.
- Laboratories exhibit a clear dominance in the market.
- The unprecedented spread of COVID-19 is a primary driver of the COVID-19 diagnostics market.
- The rapid development of advanced diagnostic technologies is reshaping the COVID-19 diagnostics market.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Grab a sample PDF of this report: https://www.imarcgroup.com/covid-19-diagnostics-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends And Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Factors Affecting the Growth of the COVID-19 Diagnostics Industry:
- Rise in Global Testing Demand:
The rapid spread of COVID-19 has created a huge demand for diagnostic testing. Governments and health organizations have launched massive testing efforts to control the virus and keep people safe. Since the virus spreads quickly, it's essential to find cases and contain outbreaks worldwide. As the virus mutates, regular testing is vital. It identifies new variants and their effects. The quest for swift and dependable testing ignites a wave of innovation. Companies are crafting cutting-edge diagnostic tools, propelling rapid market expansion. As the appetite for accuracy swells, investments in advanced testing methods surge alongside. Rapid antigen tests, real-time RT-PCR, and point-of-care solutions make testing faster and easier. These methods help us move toward a healthier future.
- Technological Advancements in Diagnostic Tools:
The rapid development of advanced diagnostic technologies is contributing to market growth. In the fast lane of innovation, companies revved up their research and development. They aimed to forge testing methods that are both swift and sharp. They developed advanced PCR techniques to meet the demand for high-throughput technologies. Automation became key, offering smooth testing solutions. Portable testing kits also became available for field use. New advances in molecular diagnostics, such as CRISPR-based detection and isothermal amplification, give healthcare professionals better tools for rapid diagnosis. These improvements make tests more accurate and faster, which reduces turnaround times. As a result, healthcare systems can respond quickly to changes in infection rates. Furthermore, progress in diagnostic technology has made at-home testing options possible, making tests more accessible and allowing individuals to monitor their own health.
- Government and Healthcare Initiatives:
Supportive government policies and healthcare initiatives are boosting the market. Many countries have set aside significant funds for healthcare infrastructure and research. This promotes the development and distribution of diagnostic tools. The collaboration between governments, private organizations, and research institutions has greatly accelerated testing services. Regulatory agencies are rapidly approving new diagnostic tools through emergency use authorizations, which speeds up their rollout. The urgent need for effective contact tracing highlights the importance of a strong diagnostic framework. This partnership, combined with proactive regulation, allows new diagnostic solutions to flourish in our healthcare system.
Leading Companies Operating in the Global COVID-19 Diagnostics Industry:
- Abbott Laboratories
- Becton Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories
- Danaher Corporation
- F. Hoffman-La Roche Ltd. (Roche Holding AG)
- Luminex Corporation
- PerkinElmer Inc.
- Quest Diagnostics Incorporated
- Robert Bosch GmbH
- Seegene Inc.
- Siemens Aktiengesellschaft
- Thermo Fisher Scientific Inc.
COVID-19 Diagnostics Market Report Segmentation:
Breakup By Product Type:
- Reagents and Kits
- Instruments
- Others
Instruments represent the larger segment due to their essential role in conducting accurate and high-throughput COVID-19 testing.
Breakup By Sample Type:
- Nasopharyngeal Swabs
- Oropharyngeal Swabs
- Nasal Swabs
- Blood
- Others
Nasopharyngeal swabs hold the biggest market share as they are the most reliable and widely used sample type for detecting respiratory viruses like COVID-19.
Breakup By Test Type:
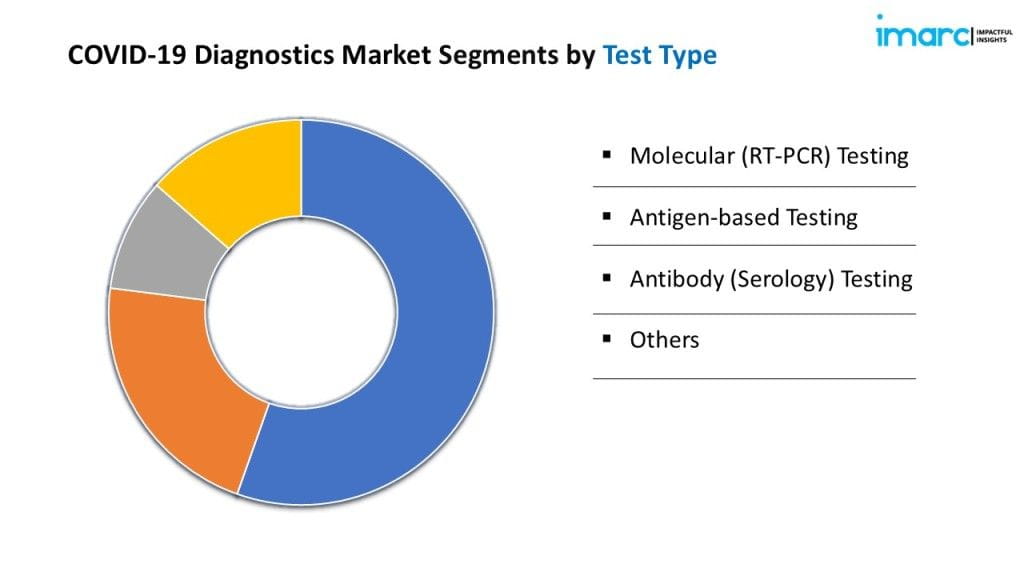
- Molecular (RT-PCR) Testing
- Antigen-based Testing
- Antibody (Serology) Testing
- Others
Molecular (RT-PCR) testing accounts for the majority of the market share because of its high accuracy and status as the gold standard for COVID-19 detection.
Breakup By Mode:
- Point-of-Care (PoC)
- Non-Point-of-Care (Non-PoC)
Non-point-of-care (non-PoC) represents the leading segment owing to the widespread use of centralized lab testing for mass and large-scale diagnostic processing.
Breakup By End Use:
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Others
Laboratories exhibit a clear dominance since they are equipped to handle extensive testing with the necessary equipment and trained personnel.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position attributed to strong healthcare infrastructure, significant investments in diagnostic technologies, and high testing rates.
Research Methodology:
The report employs a comprehensive research methodology, combining primary and secondary data sources to validate findings. It includes market assessments, surveys, expert opinions, and data triangulation techniques to ensure accuracy and reliability.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145