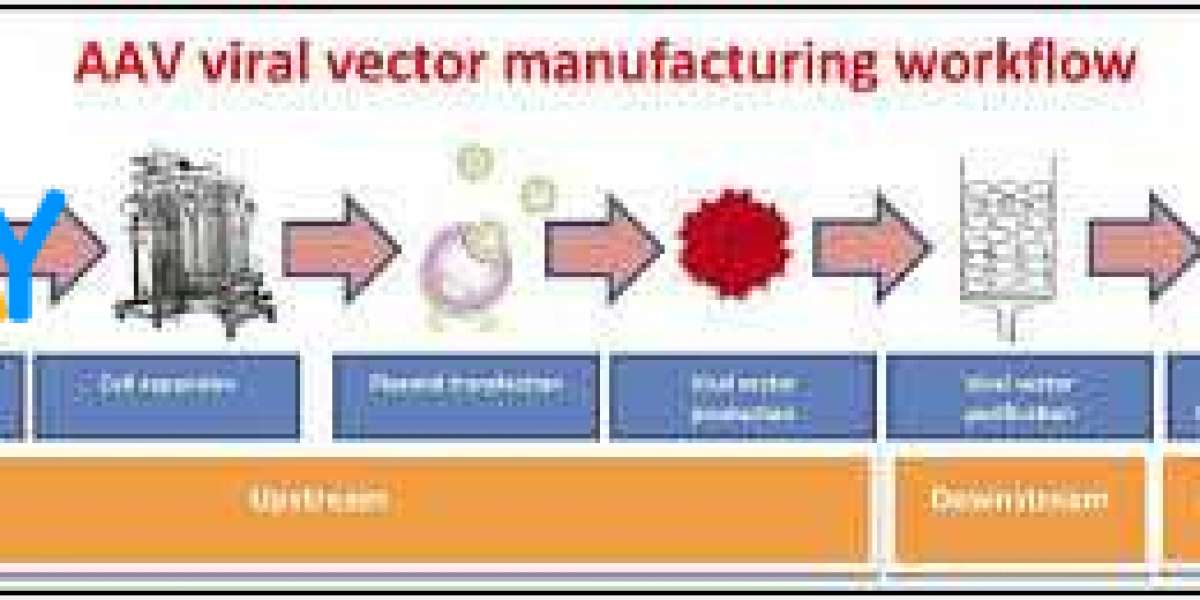
Viral vectors are engineered viruses used in genetic engineering, gene therapy, and vaccine development. These vectors are modified to carry specific genetic material without causing disease. The production of viral vectors is a critical process in biotechnology, enabling groundbreaking treatments for a wide range of diseases, including genetic disorders, cancers, and viral infections. Let’s take a closer look at how viral vectors are produced and their importance in modern medical research.
What Are Viral Vectors?
Viral vectors are viruses that have been altered in the laboratory to deliver genetic material into a host cell. While the original virus might have caused diseases, these modified versions are harmless and only used for therapeutic purposes. By utilizing viral vectors, scientists can introduce specific genes into the body to correct genetic defects or activate immune responses against diseases.
Key Steps in Viral Vector Production
The process of producing viral vectors involves several essential steps, ensuring that the final product is both effective and safe for use in medical treatments.
1. Selecting the Right Virus
The first step in viral vector production is choosing an appropriate virus. Different viruses are suited for different purposes. For instance, adenoviruses are commonly used in vaccine development because they can infect a wide range of human cells, while retroviruses and lentiviruses are favored for gene therapy due to their ability to integrate into the host cell’s DNA.
2. Genetic Modification of the Virus
After selecting the virus, the next step involves genetic modification. This process involves removing the harmful production of viral vectors genes that could cause disease and replacing them with therapeutic genes. The goal is to ensure that the virus can deliver the desired genetic material into the target cells without causing any adverse effects.
3. Preparing the Host Cells
Once the virus has been modified, it needs to be produced in large quantities. To do this, scientists use host cells — usually cells that are genetically engineered to support the replication of the modified virus. Common cell lines include human embryonic kidney cells (HEK293) and insect cells. These cells provide the machinery for the virus to replicate and produce viral particles.
4. Amplifying the Virus
In this stage, the modified virus is introduced into the host cells, where it begins to replicate. The host cells act as factories, making large quantities of the viral vectors. Once enough viral particles have been produced, they are harvested from the cells. This process requires careful monitoring to ensure that the viral vectors are growing efficiently.
5. Purification of the Viral Vectors
After amplification, the next critical step is the purification of the viral vectors. This process removes any impurities, such as residual host cells or byproducts from the replication process, ensuring that only the viral vectors are present. Purification is typically carried out using various techniques, such as filtration or chromatography.
6. Quality Control and Testing
Before viral vectors can be used in medical applications, they undergo strict quality control testing. This ensures that the vectors contain the correct genetic material, are free from contaminants, and are capable of efficiently delivering the therapeutic genes to the target cells. Rigorous testing helps to ensure the safety and efficacy of the viral vectors.
Applications of Viral Vectors
Viral vectors have diverse applications, with some of the most prominent being:
- Gene Therapy: Viral vectors are used to treat genetic disorders by introducing functional genes into a patient’s cells to correct genetic mutations.
- Vaccine Development: Certain vaccines, such as those developed for COVID-19, utilize viral vectors to deliver viral genes that stimulate an immune response.
- Cancer Treatment: Viral vectors can be engineered to target cancer cells, either delivering therapeutic genes or stimulating an immune response to attack the tumor.
Challenges in Viral Vector Production
While viral vectors hold great promise, producing them at scale presents challenges. The process can be time-consuming and expensive, with risks of contamination or inefficiency during production. Additionally, ensuring safety is crucial, as any unintended immune reactions or issues with gene integration can lead to complications.
Conclusion
The production of viral vectors is a vital component of modern biotechnology, playing a key role in gene therapy, vaccine development, and cancer treatments. With continued advancements in technology and techniques, the production of viral vectors is expected to become more efficient, making these therapies more accessible and effective in treating a wide array of diseases.