Central to these advances are tumor models—innovative systems used to mimic the behavior, progression, and treatment response of cancer cells. These models are at the heart of understanding the complexities of cancer biology and developing personalized therapies.
What Are Tumor Models?
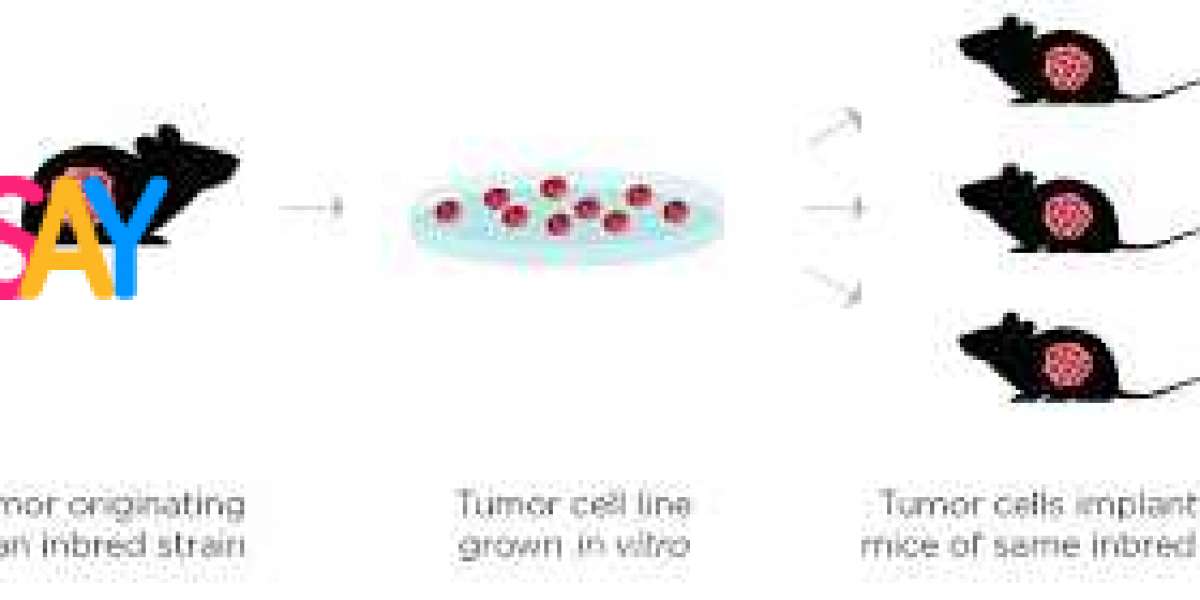
Tumor models are experimental systems that replicate the environment of human tumors. They can range from simple cell cultures to complex animal models, each designed to simulate specific aspects of cancer behavior. By studying these models, scientists can observe tumor growth, metastasis, and interactions with the immune system in controlled settings.
The two main types of tumor models include:
- In Vitro Models: These are cell-based systems grown in laboratory dishes. They allow researchers to study cancer cell proliferation, drug resistance, and molecular pathways in a controlled environment.
- In Vivo Models: These involve living organisms, such as mice, engineered to develop tumors. They provide insights into how cancer behaves in a biological system, including its response to therapies.
Advancements in Tumor Modeling
Recent innovations have transformed tumor models, making them more accurate and representative of real-world conditions.
Patient-Derived Xenografts (PDX):
PDX models involve implanting tumor tissues from patients into immunodeficient mice. These models retain the genetic and molecular characteristics of the original tumor, making them invaluable for testing personalized treatment options.3D Organoids:
Organoids are three-dimensional structures grown from patient-derived cells. They replicate the architecture and microenvironment of tumors more closely than traditional cell cultures. Organoids are particularly promising for testing drug efficacy and studying tumor-immune interactions.Genetically Engineered Models (GEMs):
GEMs involve altering the DNA of animals to express cancer-driving mutations. These models are useful for studying the progression of specific cancer types and testing targeted therapies.Tumor-on-a-Chip Technology:
This cutting-edge technology uses microfluidic devices to create miniature tumor environments. Tumor-on-a-chip systems enable high-throughput drug screening and a better understanding of how cancer interacts with blood vessels and surrounding tissues.
Tumor Models and Personalized Medicine
One of the most exciting applications of tumor models is in the field of personalized medicine. By using patient-derived cells or tissues to create models, researchers can test a range of therapies and identify the most effective treatment for an individual. This approach not only improves outcomes but also reduces the trial-and-error process of cancer treatment.
Challenges and Future Directions
While tumor models have revolutionized cancer research, they are not without limitations. For example, animal models often fail to fully replicate the complexity of human tumors, leading to discrepancies between preclinical and clinical results. Additionally, creating and maintaining advanced models like organoids or tumor-on-a-chip systems can be resource-intensive.
To address these challenges, researchers are focusing on:
- Improving the scalability of advanced models.
- Integrating artificial intelligence and machine learning to analyze large datasets from tumor models.
- Enhancing collaboration between academic, clinical, and pharmaceutical sectors to accelerate drug development.
Conclusion
Tumor models are indispensable tools in the fight against cancer. As these models become more sophisticated, they bring us closer to understanding the intricacies of cancer biology and developing treatments tailored to each patient. With continued innovation, tumor models could be the key to unlocking a future where cancer is not only treatable but preventable.