Introduction
RNA interference (RNAi) is a biological process in which RNA molecules inhibit gene expression or translation by neutralizing targeted messenger RNA (mRNA) molecules. Discovered in the late 1990s, RNAi has revolutionized our understanding of gene regulation and opened new avenues in therapeutics, agriculture, and biotechnology. This article explores the mechanisms underlying RNAi, its applications, and its implications for science and medicine.
Mechanisms of RNA Interference
Discovery and Overview
The discovery of RNAi originated in studies on gene silencing in plants and nematodes. In 1998, Andrew Fire and Craig Mello demonstrated that introducing double-stranded RNA (dsRNA) into the nematode Caenorhabditis elegans could silence genes with complementary sequences. This groundbreaking work earned them the Nobel Prize in Physiology or Medicine in 2006.
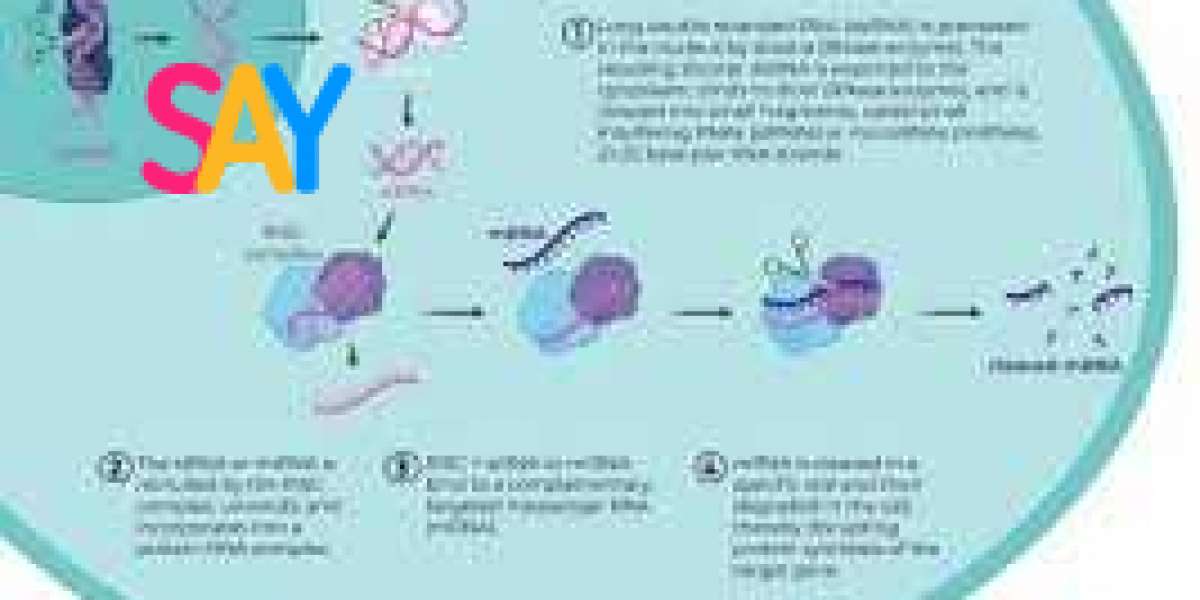
RNAi is now recognized as a highly conserved process across eukaryotes. Its core mechanism involves the degradation of mRNA, preventing the synthesis of proteins encoded by specific genes. This is achieved through two main types of small RNA molecules: small interfering RNA (siRNA) and microRNA (miRNA).
siRNA and miRNA
Small Interfering RNA (siRNA): siRNAs are typically derived from exogenous dsRNA introduced into the cell through viral infection or experimental means. These dsRNA molecules are processed by an enzyme called Dicer into short double-stranded fragments approximately 20-25 nucleotides long. One strand of the siRNA, the guide strand, is incorporated into the RNA-induced silencing complex (RISC), where it pairs with complementary mRNA. This pairing triggers the cleavage and degradation of the target mRNA, effectively silencing the gene.
MicroRNA (miRNA): miRNAs are endogenous, non-coding RNAs transcribed from the genome. They are initially synthesized as primary miRNAs (pri-miRNAs) and processed into precursor miRNAs (pre-miRNAs) before being exported to the cytoplasm. There, Dicer trims them into mature miRNAs, which are incorporated into RISC. Unlike siRNAs, miRNAs often bind imperfectly to their target mRNAs, leading to translational repression rather than direct degradation.
Key Proteins and Steps
Dicer: An endoribonuclease that processes dsRNA or pre-miRNA into siRNA or miRNA.
RISC (RNA-Induced Silencing Complex): A multiprotein complex that mediates the binding of guide RNA to target mRNA and facilitates gene silencing.
Argonaute: A core component of RISC that binds to the guide RNA and cleaves the complementary mRNA.
Applications of RNA Interference
RNAi has vast applications in various fields, from functional genomics to therapeutics and agriculture. Below are some of the most impactful areas:
1. Functional Genomics
RNAi is a powerful tool for studying gene function. By selectively silencing specific genes, researchers can observe the resulting phenotypic changes, providing insights into the gene’s role in biological processes. Genome-wide RNAi screens are widely used to identify genes involved in cell signaling, development, and disease.
2. Therapeutics
The potential of RNAi in treating diseases lies in its ability to target and silence disease-causing genes. Some key therapeutic applications include:
Genetic Disorders: RNAi can be used to silence mutant genes responsible for diseases such as Huntington’s disease and certain types of amyotrophic lateral sclerosis (ALS).
Cancer: Many cancers are driven by the overexpression of oncogenes. RNAi can downregulate these genes, potentially halting tumor growth.
Viral Infections: RNAi-based therapies can target viral RNAs, reducing the replication of viruses like HIV, hepatitis B, and SARS-CoV-2.
Approved Therapies: In 2018, the first RNAi-based drug, patisiran, was approved for treating hereditary transthyretin-mediated amyloidosis (hATTR).
3. Agriculture
RNAi has been harnessed to improve crop yield, resistance to pests, and tolerance to environmental stress. For example:
Pest Control: RNAi can target essential genes in pests, providing a species-specific method of pest control without harming beneficial organisms.
Disease Resistance: Crops can be engineered to silence genes involved in susceptibility to pathogens.
Improved Traits: RNAi has been used to produce decaffeinated coffee and hypoallergenic peanuts.
4. Biotechnology
In biotechnology, RNAi is used to engineer cell lines for the production of biopharmaceuticals, study protein interactions, and develop animal models of disease.
Challenges and Limitations
Despite its promise, RNAi faces several challenges that must be addressed for broader implementation:
1. Delivery Mechanisms
Efficient delivery of RNAi molecules to target cells remains a significant hurdle, particularly for therapeutic applications. Delivery systems such as lipid nanoparticles and viral vectors have been developed, but challenges like off-target effects and immune activation persist.
2. Off-Target Effects
RNAi can inadvertently silence non-target genes with similar sequences, leading to unintended consequences. Strategies to improve sequence specificity, such as chemical modifications to siRNAs, are under development.
3. Stability of RNA Molecules
RNA molecules are inherently unstable and susceptible to degradation by nucleases. Advances in chemical modifications and encapsulation techniques have improved their stability.
4. Immune Activation
The introduction of RNA molecules can activate the innate immune system, leading to inflammation. Designing RNA molecules that evade immune detection is an ongoing area of research.
Ethical and Environmental Considerations
RNAi-based technologies, particularly in agriculture, raise ethical and environmental concerns. For instance, the release of RNAi-engineered crops or pesticides into the environment could have unintended ecological consequences. Rigorous risk assessments and regulatory frameworks are essential to ensure their safe use.
In therapeutics, ethical considerations include equitable access to RNAi-based treatments and the potential misuse of the technology for non-therapeutic purposes.
Future Directions
The field of RNAi continues to evolve, with several promising developments on the horizon:
Improved Delivery Systems: Advances in nanotechnology and viral vectors are expected to enhance the delivery of RNAi molecules to specific tissues and cells.
CRISPR and RNAi Synergy: Combining RNAi with CRISPR-based gene editing could offer more precise and efficient ways to regulate gene expression.
Expanded Therapeutic Applications: Ongoing research aims to apply RNAi to treat a broader range of diseases, including neurodegenerative disorders, metabolic diseases, and rare genetic conditions.
Agricultural Innovations: RNAi-based pesticides and stress-tolerant crops are likely to play a significant role in sustainable agriculture.
Conclusion
RNA interference has transformed our understanding of gene regulation and holds immense promise for various scientific and practical applications. While challenges such as delivery, specificity, and stability remain, ongoing advancements in technology and research are addressing these hurdles. As RNAi continues to mature, it has the potential to revolutionize medicine, agriculture, and biotechnology, making it one of the most exciting areas of modern biology.